FIlling Equipment
Filling Equipment for Bio-Similar Development
With many of the blockbuster biologic drugs of the ’90s are coming off of patent, companies are racing to provide a clinically similar alternative to these pioneering products. Like any biologic drug, they require care and attentiveness throughout the manufacturing process. Therefore, it is necessary to maintain aseptic conditions throughout the production process of these drug products.
AST aseptic filling products address the unique biosimilar processing requirements to gently care for biologic products during the fill-finish for Biosimilars process. Our highly accurate peristaltic dosing system uses single-use pharmaceutical grade tubing that has been optimized to keep shear stress to an absolute minimum.
Temperature controlled filling is also possible with the ASEPTiCell® and GENiSYS® systems. We have experience dosing products at temperatures as low as 3°C.
In the Know
High yield, High-Value
Achieving high yield is absolutely critical with biologic products, because of their extremely high-value. To ensure that every container is properly dosed AST integrates extremely sensitive electronic balances with our system that provide an accurate in-process measurement of the volume within the containers. The balances can provide real-time feedback to re-dose low filled containers to minimize any wasted products. The GENiSYS® Lab Container Filling System is the only tabletop filling system available on the market with this integrated fill weight verification feature.

Biologics & Pre-Filled Syringes
Pre-filled syringes and combination devices also seem to be a common requirement for biosimilars development equipment. Many biotechnology companies are gravitating toward silicone-free syringe barrel options and desire to eliminate the oxygen bubble commonly trapped between the syringe piston and the liquid product. AST has a patented process called multi-stage vacuum piston insertion that is ideal for use with silicone-free syringes like West Pharmaceuticals CZ syringes. This process pulls vacuum from within the syringe to remove the oxygen and then gently places the piston into its final location.
AST has the expertise and process know-how to address the fill-finish challenges of your biologic product.
On-Board AST as your bio-similar development partners
Request a consultation and a team member will contact you soon! We strive to provide an exceptional customer experience by providing innovative solutions.
Related Content

The Advantages Of Robotics In Aseptic Fill Finish
AST’s latest whitepaper on robotics, now available for download.
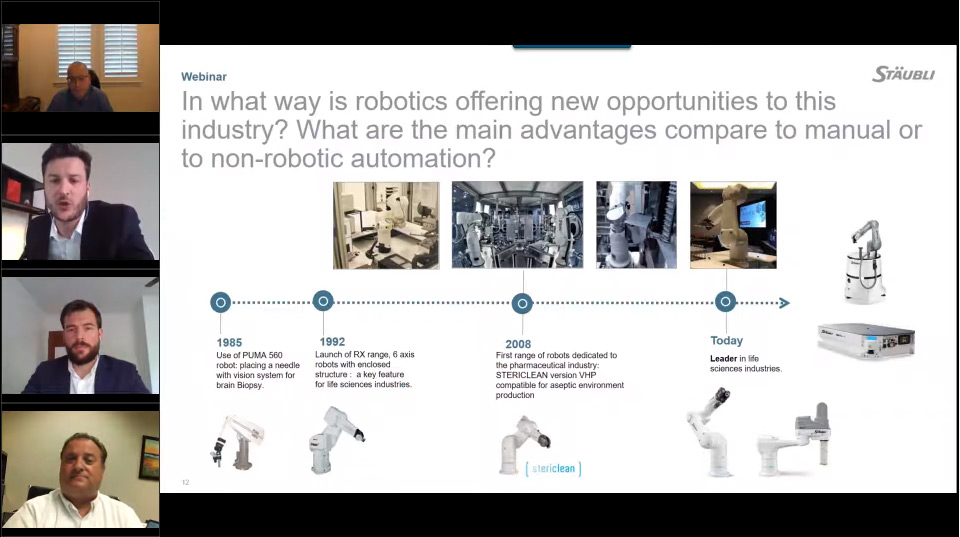
How Robotics Offer New Answers to Today’s Challenges
Staubli presents a panel discussion webinar on robotics with Joe Hoff

