Archives for paul@extractmg.com Page 2
For pharmaceutical manufacturers, every endeavor into drug development comes with a measure of uncertainty and unpredictability. It’s important to have a plan in place for when the need for adaptability arises, and that leaves many looking into proven ways to incorporate flexibility into their operational strategy. Additionally, trends in aseptic fill-finish manufacturing show customers are […]
Pharmaceutical manufacturing continues to see growth in the field of highly targeted, high-value liquid pharmaceuticals. Increasingly, entities like CDMOs, are looking at the most effective ways to implement adaptable, streamlined multi-modal operations designed for uptime and time-to-market optimization. In the initial phases of planning your aseptic fill-finish operation, there are many decisions to make both […]
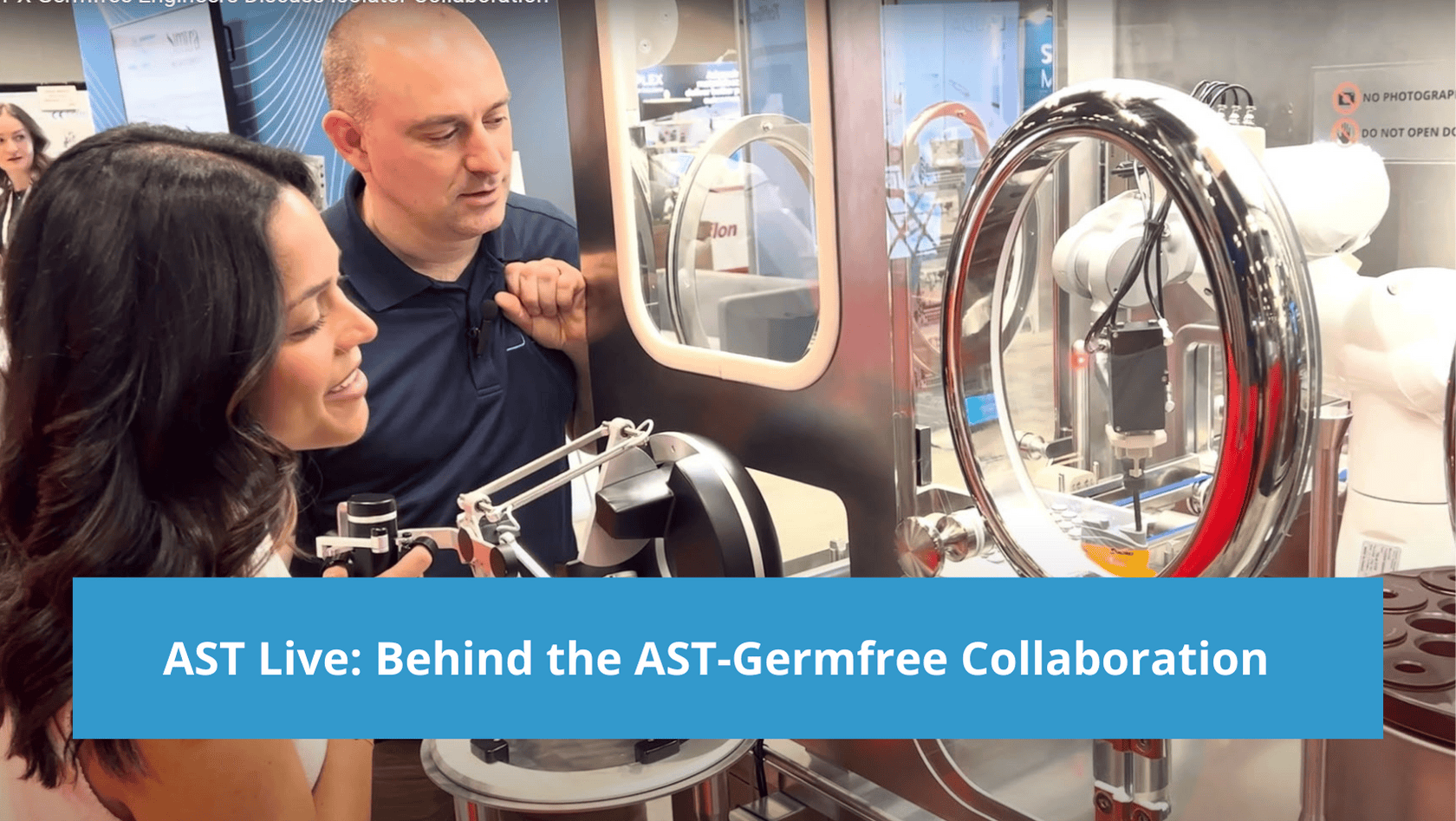
Meet two of the innovative minds that helped lead the AST-Germfree collaboration on next-generation barrier technology: Engineers Michael Moore (AST) and Paula Rizo (Germfree) connected at Interphex to discuss what sets this isolator solution apart and what the journey over the course of this collaboration has been like for the two teams. At Interphex 2024, AST was thrilled to […]
The following article is part 2 of a blog series that has been adapted from AST’s VP of Sales and Marketing Josh Russell’s recent article in Cleanroom Technology. In part 1, we discussed considerations for isolator configurations and AST’s approach to integrating automation in our new barrier technology solution. The most important questions to consider […]
The following 2-part blog series has been adapted from AST’s VP of Sales and Marketing Josh Russell’s recent article in Cleanroom Technology.
The AST team recently returned from New York City, the site of Interphex 2024, and to say we came back motivated would be an understatement. The show was teeming with enthusiasm as life sciences professionals from across the globe gathered in the Big Apple for the premier pharmaceutical and biotechnology event. The stage was set […]
In the fill-finish manufacturing of parenteral liquid pharmaceuticals, maintaining product sterility is paramount. The choice of what kind of barrier technology to utilize becomes key, as operations must account for the production environment and the level of control and efficiency needed to hit key production milestones. For an increasing amount of industry professionals, isolators are […]
One of the more challenging, essential aspects of planning for an isolated fill-finish operation is determining the approach to decontamination. Sterility assurance is a top priority, and ensuring a selected method meets regulatory thresholds is vital.
When AST produced its first fill-finish solution in 2006, two key convictions drove that early innovation: A commitment to imagining a never-before-seen aseptic multi-format processing system that was versatile and flexible, and a desire to produce robust, American-made aseptic fill-finish products. Ahead of the release of our new isolator, we wanted to explore our commitment […]
**For Immediate Release** Oviedo, Florida, and Tacoma, Washington, February 5, 2024 – CURIS System, an innovator in advanced decontamination technology, and AST, a leader in aseptic fill-finish processing technology, today announced a groundbreaking partnership. This collaboration marks a significant step forward in the pharmaceutical manufacturing industry, as the two companies combine technology to revolutionize the […]