Archives for paul@extractmg.com Page 3
When AST came to the table on the design for our new fill-finish isolator, one of the first areas AST engineers wanted to address was the usability and accessibility of the isolator and corresponding operations. With our customers’ point of view in mind, we wanted to address specific points of friction routinely encountered by operators […]
By Steven Ng, CTO & VP of Customer Service This past October, AST announced a groundbreaking collaboration with Germfree on a new fill-finish isolator. This exciting announcement was the result of just over a year of brainstorming, research, and development, as our team at AST set out to create an isolator solution that would meet […]
The path to a successful life science startup is exciting, difficult, and unpredictable. Recent years have seen a volatile pattern emerge of challenge, change and innovation within the life sciences. The trend towards innovative pharmaceutical solutions is projected to continue into 2024, as breakthroughs in treatment modalities, supply chain management and patient-centered medicine are tracking […]
Aseptic fill-finish professionals are continually looking to augment their approach to a market that continues to grow and reflect innovations happening around sterile parenteral treatments. When considering the flexibility of an operation, it’s important to investigate every aspect of the fill-finish process. Flexibility applies to any process or technical optimization that quickens scalability and removes […]
Josh Russell gives a live demonstration of AST’s isolated GENiSYS C as part of Virtual Pharma Expo 2023.
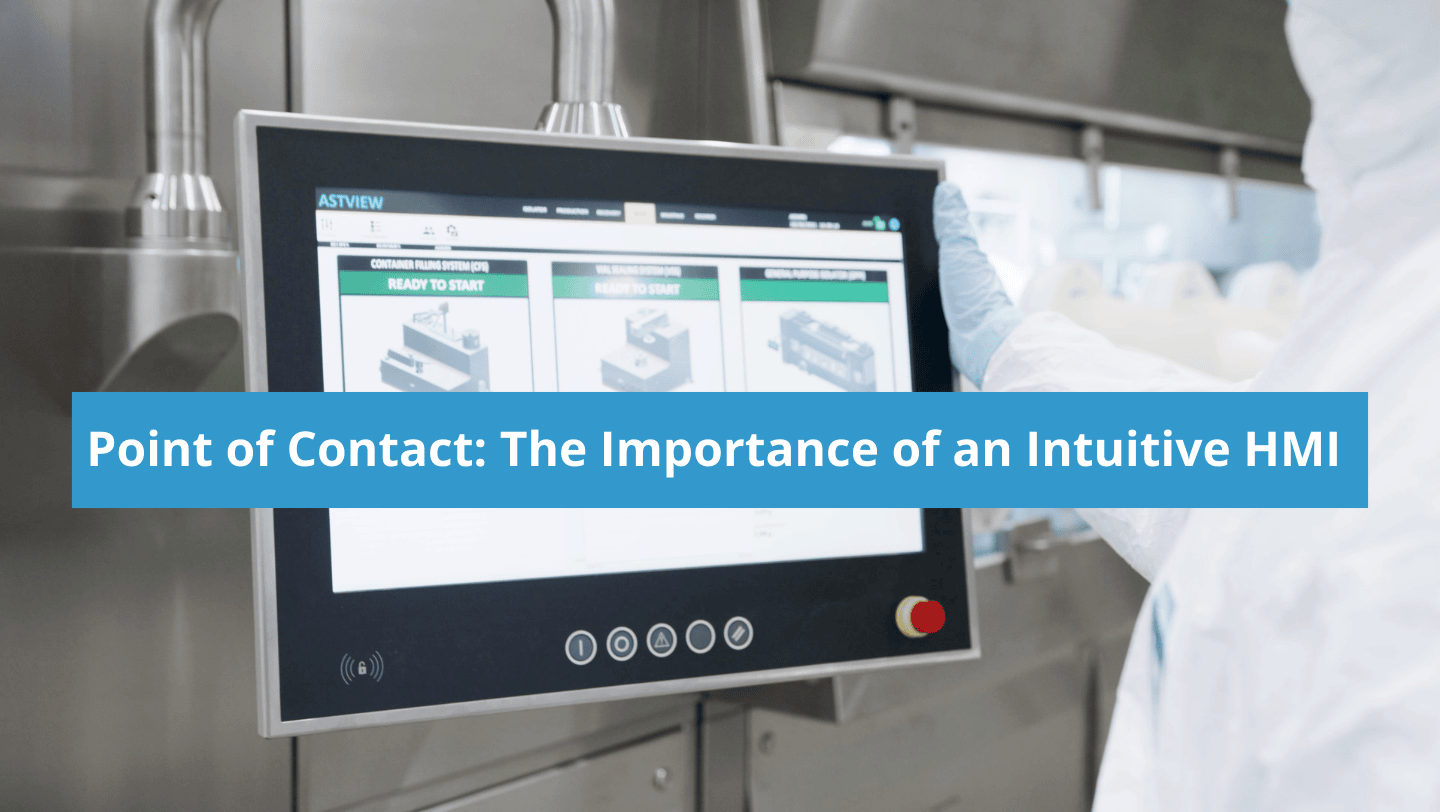
As aseptic fill-finish technology continues to make strides towards broader use of robotics and automation, the usability and performance of a system’s Human Machine Interface (HMI) becomes vital to the operator. Considering the amount of complexity native to aseptic processing and the variables possible in any given system, including format, finishing, and barrier options, fill-finish […]
A Word from AST President & CEO Joe Hoff It’s common practice at AST to revisit our Mission Statement as we go about our work to provide quality fill-finish solutions to our customers. That mission—to enhance the reliability, productivity and safety of aseptic processing by offering intelligent, intuitive, and innovative solutions—informs every aspect of what […]
For small-batch, high-value drug products, the aseptic advantages of pre-sterilized ready-to-use vials are clear. To realize the full scope of benefits that RTU vials can provide, integration with traditional processes and equipment must be carefully considered. Modern and flexible technologies such as robotics are key to seamlessly integrating RTU vials into aseptic fill/finish processes. The […]
Throughout the lifecycle of a drug product, many critical target points for optimization make all the difference in the time-to-market equation. A holistic approach to the drug development process that’s able to anticipate challenges in production, regulatory requirements, and manufacturing practices should be considered. A key component of this approach is establishing early and clear […]
Small-batch manufacturing of liquid pharmaceuticals is slated for exponential growth in the coming years and represents exciting possibilities for the future of medicine and personalized healthcare. Whether biologics, biosimilars, or orphan drugs developed in the fight against rare diseases, technology is providing solutions for the manufacturing challenges traditionally associated with small-batch aseptic processing and consequently […]