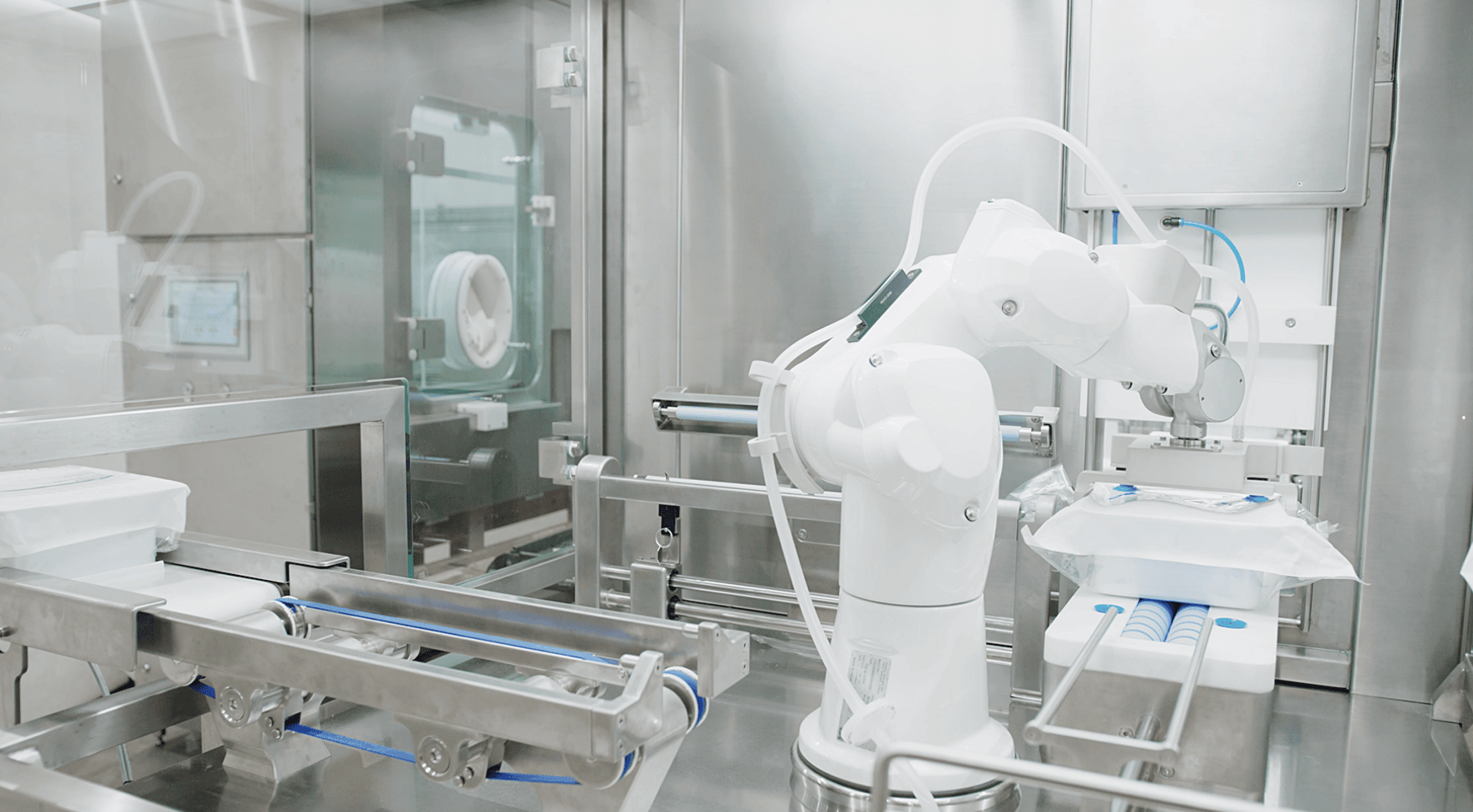
Throughout the lifecycle of a drug product, many critical target points for optimization make all the difference in the time-to-market equation. A holistic approach to the drug development process that’s able to anticipate challenges in production, regulatory requirements, and manufacturing practices should be considered. A key component of this approach is establishing early and clear tech transfer strategies.
The considerations of scaling an operation are numerous: increased batch sizes, recipe changes, risk control strategies applicable at every stage, and ensuring process information remains uncorrupted and highly repeatable, all under a regulatory framework that requires stringent validation protocols and product quality standards. These factors require a high amount of foresight and planning, and early adoption of comprehensive technology transfer protocols coupled with the right fill-finish systems is critical.
Granted, during the clinical/pre-clinical stage of drug development, it can be hard to imagine the end of the drug development lifecycle, which is ultimately the full R&D process concluding with in-house distribution or selecting a capable CDMO partner for the commercial realization of a product. In either case, it’s vital to understand the specific nature of the product, anticipate any regulatory or procedural challenges that lie ahead, and identify possible solutions to answer those challenges. How much of a difference would it make, for example, if the benchtop system used in preliminary R&D work provided both the automated solutions that could scale through commercial production and the recipe parameters, system features, and servo control needed to accurately simulate mass production operations?
This type of direct tech transfer between machines allows manufacturing to scale with minimal friction. When key technologies like HMIs, pumps, sterility protocols, IPC methods, and fill and closing mechanisms are able to transfer seamlessly from system to system, it reduces the need for revalidation and saves valuable time over the life of an operation. Fill-finish solutions with these capabilities prime the treatments of tomorrow for success.
Operational Differentiators for Fill-Finish Manufacturing
Cost: Selecting a fill-finish solution that is technologically suited to the regulatory and procedural demands of the drug development process is a valuable way to protect resources invested in a project. The time saved alone from having a simplified, modular system with minimal change parts is substantial as downtime is costly (an hour of manufacturing downtime can cost upwards of $250,000). An automated, robotic fill-finish system, though a more significant upfront investment, can carry the same parts and functionality through the scale-up process. This will ultimately provide a larger ROI due to less downtime, less additional equipment, and more stable, repeatable conditions for high-value liquid pharmaceuticals, leading to less product loss.
Speed: Speed-to-market is a major differentiator in any product development operation and should be planned for in any implementation of a technology transfer strategy. In an automated setting, costly and time-involved manual methods can be avoided at nearly every stage of the fill-finish process, from data logging to closing and sealing a container. Automation can likewise reduce human interventions, which are no friend to timely production. A modular approach with tool-less change parts also means that operations can avoid wasting valuable time with equipment changeover.
Compliance: One of the most challenging and necessary aspects of tech transfers is maintaining regulatory compliance all the way through the process. Any number of changes or developments can happen during the course of R&D, so having a process oriented towards the cGMP realities of each stage of development is key to successfully launching a new product, especially when in partnership with another party and product comparability becomes an essential criterion. Automation allows for uncorrupted data traceability across the entire fill-finish progression, which is critical for preserving process knowledge for a successful technology transfer. ASTView is one such solution that fully integrates with AST’s aseptic fill-finish systems and is able to collect all critical in-process parameter data, allowing the user to export the data for analysis and optimization. Each system can also be upgraded with an Electronic Batch Record (EBR) system to provide 21 CFR Part 11 compliance.
Strong tech transfer strategies are foundational to any early R&D project looking to scale, and with flexible, automated solutions, the challenges and parameters of each stage of the drug development lifecycle can be navigated with confidence.