The last four years have seen remarkable developments in the field of cell and gene therapy. In 2019, the FDA forecasted that the US is likely to add 10-20 cell and gene therapies per year until 2025, and current prognostication has development tracking at that speed. There has never been a brighter time for potential treatments for rare and life-threatening diseases, as modern cell & gene science looks to provide the exact patient-centered protocols to make a difference. Advancements like these are what make the world of R&D so compelling. It was only seven years ago that the first CAR T treatment was approved and now, as of 2023, there are six different FDA-approved CAR T therapies cleared to treat a growing number of cancers. More broadly, 222 ATMPs were put into clinical trials globally just last year. The potential of cell and gene therapies is clear, and the groundbreaking wave of treatments is only expected to keep building.
The APIs commonly used to build cell and gene therapies include:
- Oligonucleotides – nucleic acid-based therapies that are typically focused on gene silencing and have potential to treat a wide range of diseases.
- Peptides – short-chain amino acids that can be synthesized and applied for a variety of R&D purposes, including drug development, cancer treatments, and vaccine development. Cell Penetrating Peptides (CPP) can be used as vectors for a variety of cell-based treatments.
- Viral Vectors – Viruses modified for minimal risk and engineered to deliver genes to cells. Viral vectors are also used in vaccines to induce an immune response.
- mRNA – Messenger RNA is utilized with lipids to deliver instructions to the cell to create specific proteins that fight disease.
- Plasmids – A small DNA molecule, independent of chromosomal DNA, utilized in Recombinant DNA technology, able to modify genes and deliver helpful traits to the cell.
Evolving Regulatory Standards
But with a maturing market comes challenges. Regulations around cell and gene therapy are progressing as well, and though governing bodies are making attempts to optimize regulatory standards around the rapidly evolving nature of cell and gene therapy production, there are still hurdles to clear. With the new Annex 1 coming into effect, many are doing their due diligence to not only ensure that their aseptic manufacturing operations are equipped for growth but are also prepared for compliance. The combination of the unpredictable market growth rate with clarified cGMP standards creates a need for a flexible solution that can meet product requirements without delaying distribution and without regulatory lapses due to operational changeover.
Additionally, cell and gene therapeutic products themselves are highly fragile and require the most efficient aseptic fill-finish process possible. The batch size is typically very small, and the cell or gene therapy must be produced and finished quickly (within a 2 to 3-hour timeframe) due to its inability to withstand storage at room temperature.
Best Aseptic Practices for Cell & Gene Therapy Production
These stringent quality standards point to some necessary differentiators for any successful cell and gene manufacturing operation:
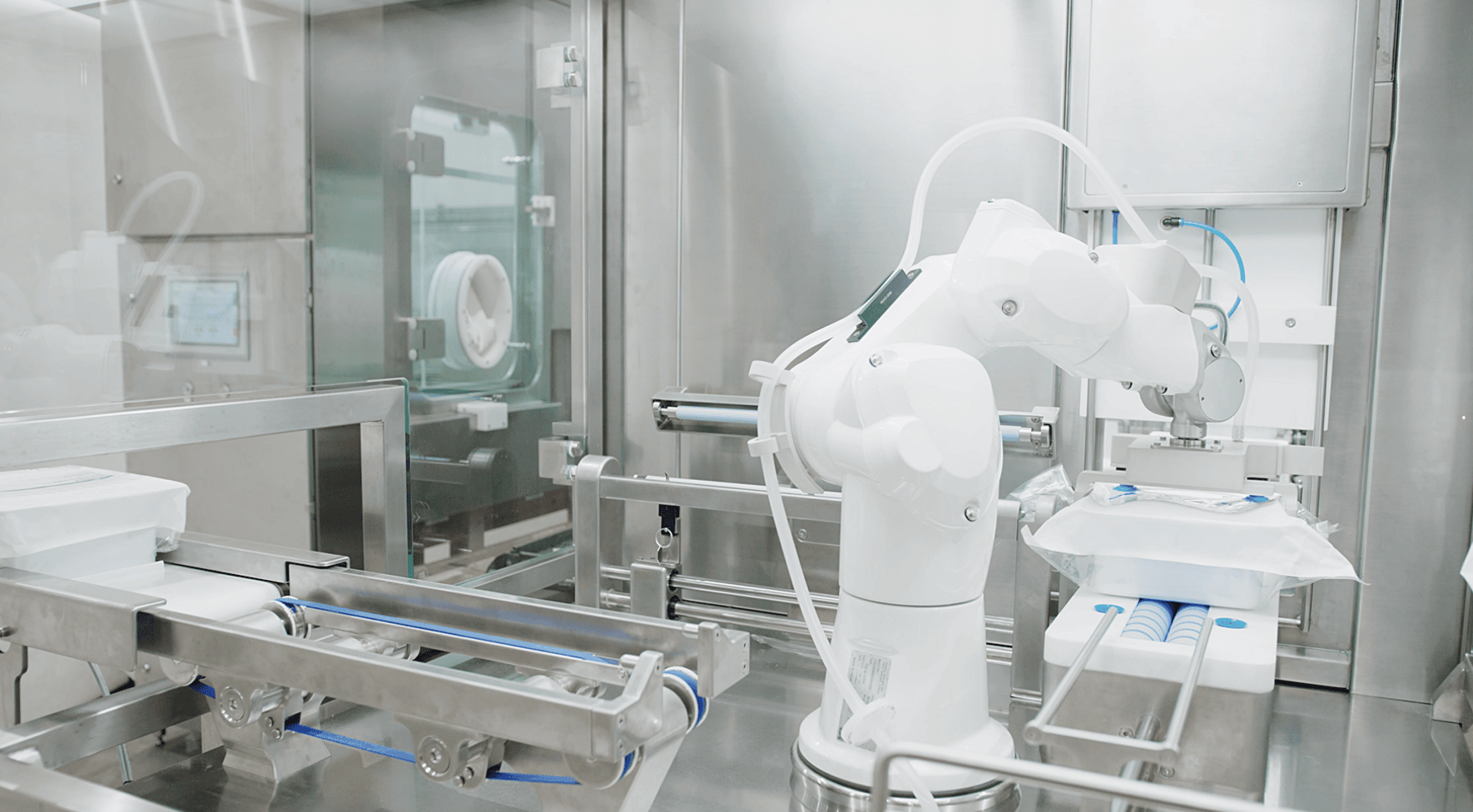
Low-Risk Automation –
Environmental factors such as vibrations, static, excess process movement, and non-bifurcated processing modules all put your liquid pharmaceutical product at risk. Automation has been shown to cut down the prevalence of human error, providing an ideal mode for producing delicate, high-value products. Automation also eliminates time lost due to recipe changeover.
Real-time Data Feedback –
Because of the highly targeted, delicate nature of producing cell and gene treatments, any viable fabrication process must include real-time data feedback to be able to monitor and make corrections as soon as issues arise. For any products looking to scale to distribution, real-time data feedback is necessary for 100 percent traceability, ensuring product quality and cGMP compliance.
Zero-Waste IPC –
Cell and Gene therapies, wherever they’re located within the product lifecycle, represent a significant investment of time, innovation, and money. Patients waiting for treatments often can’t afford delays due to product loss. Finding the best aseptic solution that enables you to steward your product all the way through the development process is vital. A zero-waste IPC approach not only protects your investment but provides a viable path towards carrying a cell and gene product to distribution.
Small Batch Production in RTU and Custom Containers –
Optimizing an operation for both a diverse range of recipes and finishing options utilizing a combination of Ready-To-Use and custom containers is key to planning and scaling for targeted, small-batch APIs. Format, non-sterilized containers lead to a loss of production time and often product loss. A flexible fill-finish option gives an operation the ability to adapt to the needs of a diverse customer base.
Simplified, Transferable Technology –
As a cell and gene therapy makes its way through the drug development progression, an important consideration is the tools and technology enlisted in the manufacturing process. For maximum yield-to-market efficiency, a simplified, modular approach that includes a low number of change parts with no tool requirements is best. Additionally, transferable technology means minimal changeover time as an operation grows.
Leveraging these solutions through semi-automated to fully automated and integrated cGMP manufacturing is foundational to the ongoing development of cell and gene therapies.
Learn how AST can help your organization navigate cell and gene therapy manufacturing for 2023 and beyond with our industry leading aseptic filling solutions – Contact our experts today!