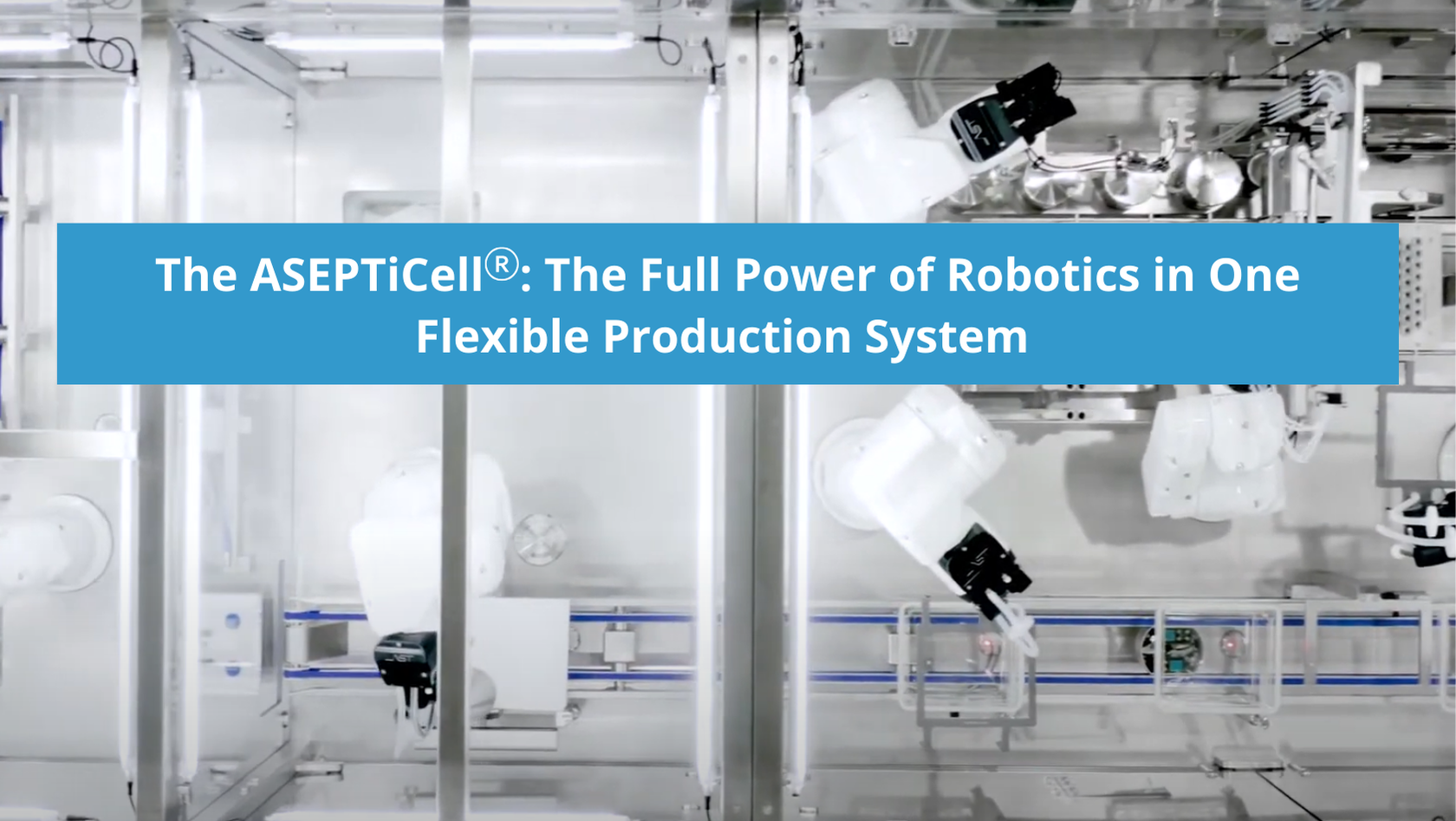
The ASEPTiCell® is where the full power of AST’s automated and robotic solutions meet precision modular engineering for the commercial production of medium-batch liquid pharmaceuticals. Fully automated from start to finish, this highly intuitive, flexible fill-finish system was the industry’s first aseptic multi-format processing machine capable of processing ready-to-use nested vials, cartridges, and syringes on the same line. The latest-generation ASEPTiCell builds on that initial innovation, offering industry-leading multi-modal flexibility, efficiency with recipe-driven production, and fewer change parts for minimal format changeover time and optimal machine uptime.
The heart of AST’s ASEPTiCell is the flexible, robotic FCM, designed for commercial-level filling and closing of liquid pharmaceuticals. It offers in-nest processing for tubs and custom nest configurations for trayed formats. The FCM leverages simultaneous filling and nest handling with no wasted motion while maintaining First Air.
The Next Tier of No-Touch Technology
The ASEPTiCell is a fully automated, no-touch solution featuring meticulously engineered robotics and automation that’s highly adaptable and programmable. Recipe-driven production allows operators to utilize the full flexibility and capability of this advanced system for a variety of ready-to-use containers. No-touch solutions should excel in three areas: de-risking aseptic processing procedures, empowering the operator, and simplified, highly repeatable automation for critical processes. Our goal at AST is to minimize the risk that humans pose to the aseptic fill-finish process while not removing the human ability to respond, troubleshoot, and resolve any issues that may arise. Robotics and automation are clear, proven methods of decreasing risk and promoting aseptic environments, both by removing the greatest source of decontamination (a human operator) and removing a significant degree of variability and unpredictability associated with manual processes. Robotics and automation also work in concert with advanced decontamination technologies, allowing for quicker machine turnover times.
AST’s robotics are designed to execute critical processes with precision and repeatability across the fill-finish line. Simplified, well-designed movements are a way to ensure that robotic handling adheres to proper aseptic technique, which is especially crucial in critical zones and for cGMP compliance, including alignment with the new Annex 1. Robotic processing in the ASEPTiCell minimizes air disturbance over exposed product and unidirectional airflow is always maintained.
The net result of this approach empowers the human role as operator while limiting physical interaction with the aseptic environment. This is also where the simplicity and intuitiveness of the human-machine interface becomes crucial. The ASEPTiCell features a fully integrated HMI (ASTView) that serves as the central control for the fill-finish, decontamination, and isolator systems for simplified, cross-technology operation. The HMI also offers an additional EBR system with complete data and systems records for 21 CFR Part 11 compliance. All-in-one touchscreen automation allows for frictionless, efficient operations.

Compact and efficiently designed:
When compared to other similar solutions, the ASEPTiCell offers a more compact, flexible solution with the same optimization for throughput and quality.
Format changeover is a crucial aspect to account for in any aseptic processing operation. Automation and robotics are great tools, but they shouldn’t have to be overly complex or laborious. AST engineers make it a point of emphasis to design fill-finish systems that have the least number of change parts possible for optimal production. In the case of the ASEPTiCell, full format changeover can be completed in just 15 minutes by a trained operator.
Higher Throughput for Commercial Production
The ASEPTiCell is the ideal solution for parenteral drug manufacturers looking for a higher throughput and larger batch-size processing. The system features a throughput of one hundred units per minute and can process batch sizes of 30,000 units or higher. For vial processing, the latest generation system comes with AST’s new VSM-100 for high-speed vial capping. Additionally, the system is available with 100 percent IPC functionality with no compromise to machine output.
When combined with the new AST isolator, The ASEPTiCell is a robust fill-finish system with industry-leading cycle times (1 hour, including aeration) and designed as a comprehensive, user-friendly turnkey solution.
Applications:
- Medium batch production of commercial and clinical materials
- Biologics, proteins, and potent products
- Filling of toxic and cytotoxic materials
- Multi-modal fill-finish manufacturing facilities.
- The full range of cGMP manufacturing applications
The ASEPTiCELL and the AST isolator offer parenteral drug manufacturers the ultimate aseptic processing system for cGMP production. Learn how AST can provide your organization with the next-generation fill-finish technology – contact our experts today.